To understand the nature of the Universe we are forced to read the message encoded in the light we receive, for which astronomers use the technique called spectroscopy. Basically, it consists in using a prism to split the light in its component colors.
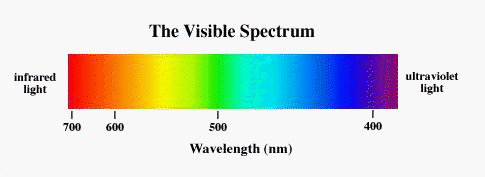
Newton (1660's) was the first to describe that the light from the Sun is made of a mixture of light
of violet, blue, green, yellow,
orange, red colors called the visible spectrum.
The Sun emits most of the light in the yellow range. Hotter stars emit more light in the blue, while
cooler stars emit more red light.

In 1810's Fraunhofer (German physicist) magnified the Sun's spectrum using a grating and discovered dark lines

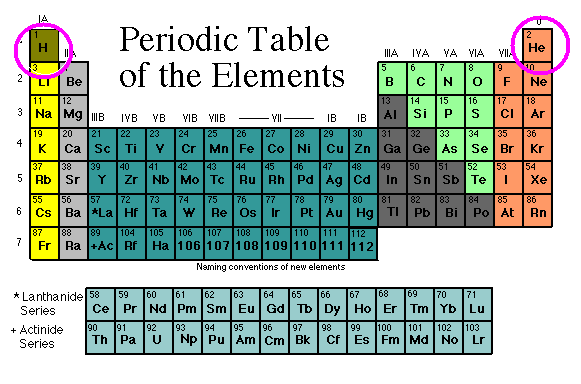
These lines are caused by atoms in the solar atmosphere that absorb light at specific places in the spectrum. Since each element produces a unique absorption pattern, the identification of such lines allows us to tell the chemical composition of a star! To date, we know that the most distant stars are made of the same elements present on Earth.
 J.J. Thompson
J.J. Thompson
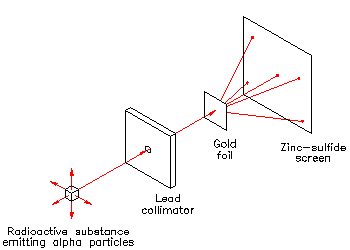
 E. Rutherford
E. Rutherford 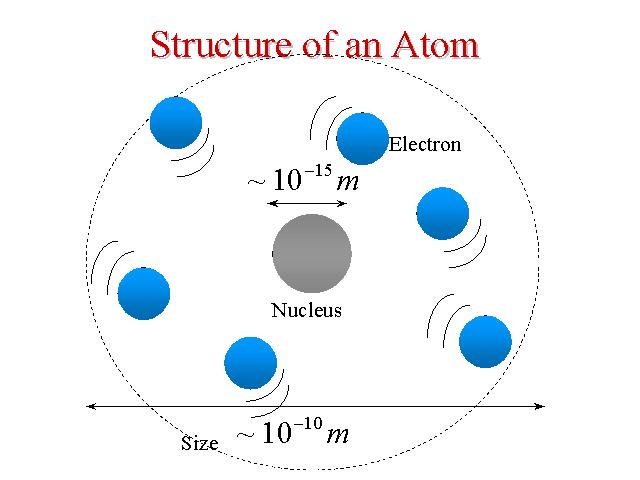
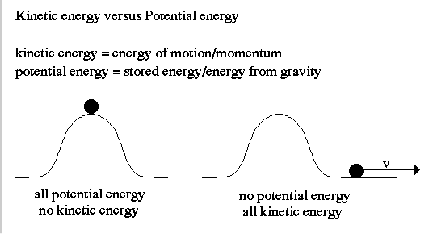
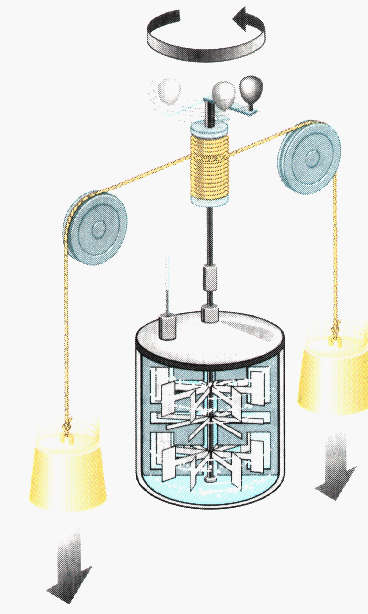 Joule's experiment
Joule's experiment