
Radioactive parent elements decay to stable daughter elements.
Radioactivity was discovered in 1896 by Henri Becquerel. In 1905, Rutherford and Boltwood used the principle of radioactive decay to measure the age of rocks and minerals (using Uranium decaying to produce Helium. In 1907, Boltwood dated a sample of urnanite based on uranium/lead ratios. Amazingly, this was all done before isotopes were known, and before the decay rates were known accurately.
The invention of the MASS SPECTROMETER after World War I (post-1918) led to the discovery of more than 200 isotopes.
Many radioactive elemtns can be used as geologic clocks. Each radioactive element decays at its own nearly constant rate. Once this rate is known, geologists can estimate the length of time over which decay has been occurring by measuring the amount of radioactive parent element and the amount of stable daughter elements.
Examples:
Radioactive Parent |
Stable Daughter |
|---|---|
Potassium 40 |
Argon 40 |
Rubidium 87 | Strontium 87 |
Thorium 232 | Lead 208 |
Uranium 235 | Lead 207 |
Uranium 238 | Lead 206 |
Carbon 14 | Nitrogen 14 |
In the above table, note that the number is the mass number (the total number of protons plus neutrons).
Note that the mass number may vary for an element, because of a differing number of neutrons.
Elements with various numbers of neutrons are called isotopes of that element.
Each radioactive isotope has its own unique half-life.
A half-life is the time it takes for half of the parent radioactive element to decay to a daughter product.
Examples:
Radioactive Parent |
Stable Daughter |
Half life |
|---|---|---|
Potassium 40 |
Argon 40 |
1.25 billion yrs |
Rubidium 87 | Strontium 87 | 48.8 billion yrs |
Thorium 232 | Lead 208 | 14 billion years |
Uranium 235 | Lead 207 | 704 million years |
Uranium 238 | Lead 206 | 4.47 billion years |
Carbon 14 | Nitrogen 14 | 5730 years |
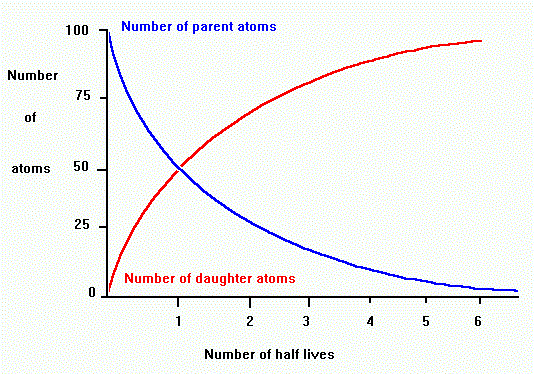
Radioactive decay occurrs at a constant exponential or geometric rate.
The rate of decay is proportional to the number of parent atoms present.

The proportion of parent to daughter tells us the number of half-lives, which we can use to find the age in years.
For example, if there are equal amounts of parent and daughter, then one half-life has passed.
If there is three times as much daughter as parent, then two half-lives have passed. (see graph, above)
Radioactive decay occurs by releasing particles and energy.
Uranium decays producing subatomic particles, energy, and lead.
As uranium-238 decays to lead, there are 13 intermediate radioactive daughter products formed (including radon, polonium, and other isotopes of uranium), and 8 alpha particles and 6 beta particles released. There are three types of subatomic particles involved:
Note that some elements have both radioactive and non-radioactive isotopes. Examples: carbon, potassium.
As seen in the tables above, there are three isotopes of uranium. Of these, U-238 is by far the most abundant (99.2739%).
Radioactive elements tend to become concentrated in the residual melt that forms during the crystallization of igneous rocks. More common in SIALIC rocks (granite, granite pegmatite) and continental crust.
Radioactive isotopes don't tell much about the age of sedimentary rocks (or fossils). The radioactive minerals in sedimentary rocks are derived from the weathering of igneous rocks. If the sedimentary rock were dated, the age date would be the time of cooling of the magma that formed the igneous rock. The date would not tell anything about when the sedimentary rock formed.
To date a sedimentary rock, it is necessary to isolate a few unusual minerals (if present) which formed on the seafloor as the rock was cemented. Glauconite is a good example. Glauconite contains potassium, so it can be dated using the potassium-argon technique.
Procedure to study:
Useful in dating:
Reheating "anneals" or heals the tracks.
The number of tracks per unit area is a function of age and uranium concentration.
Return to Online Historical Web page
Return to GSAMS Historical Geology Web page
Return to Georgia Geoscience On-line
Document created by: Pamela J. W. Gore
Georgia Perimeter College, Clarkston, GA
Document created February 4, 1996
Modified April 4, 1997
Last modified February 3, 1999